Peptide Purification: A Lab Guide
By: Clark Jones, PhD
Peptides are short chains of amino acids linked by peptide bonds. Synthetic peptides allow researchers to study a variety of pathways and functions of peptides that incorporate modifications or unnatural amino acids that cannot be obtained from natural proteins. Understanding the synthesis process, including key chemical principles, challenges, and optimization strategies, is critical for obtaining high-quality peptides suitable for research applications.
The Basics of Peptide Synthesis
Peptide synthesis is the sequential chemical linking of amino acids to form a defined chain. Each amino acid has two reactive groups: the amino group (-NH₂) and the carboxyl group (-COOH). Peptide bonds form when the amino group of one amino acid reacts with the carboxyl group of another, releasing water.
Controlling which groups react at any given step requires protecting groups and activating agents that ensure the peptide bonds form in the correct sequence without unwanted side reactions.
Two primary synthesis strategies are used in research:
Solid-Phase Peptide Synthesis (SPPS)
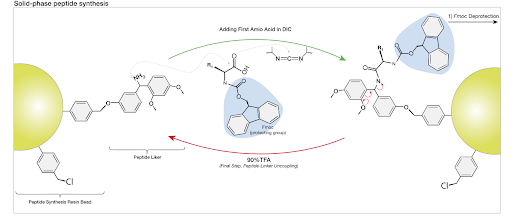
- SPPS is the most common method for research peptides. It anchors the first amino acid to an insoluble resin, allowing all subsequent reactions to occur on a solid support (1). After chain elongation, the peptide is cleaved from the resin. Key advantages of SPPS include:
- Simplified purification after each step.
- Compatibility with automation for rapid synthesis.
- Ability to incorporate modifications such as phosphorylation, acetylation, or non-natural amino acids.
- Liquid-Phase Peptide Synthesis
- In this approach, peptide chains are built in solution. This strategy is less common in modern research, but it can be useful for short peptides or large-scale production. It requires careful handling and repeated purification steps after each coupling (2).
Stepwise Workflow of Solid-Phase Peptide Synthesis
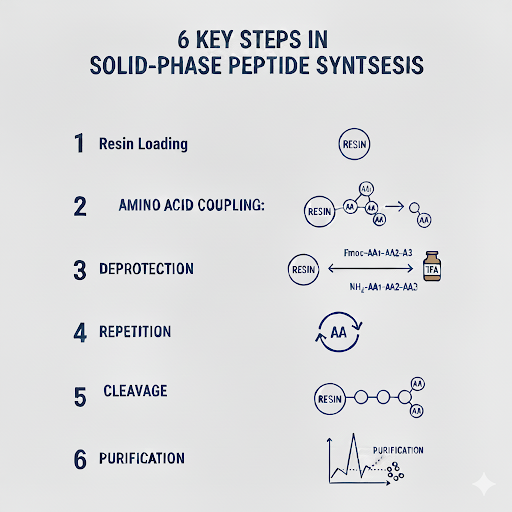
SPPS follows a repetitive cycle of deprotection and coupling for each amino acid added. The main steps are:
- Resin Loading: The C-terminal amino acid is attached to a solid resin (often through a linker that will be cleaved later). The loading density and resin type affect the yield and purity of the desired product.
- Amino Acid Coupling: The new amino acid is protected using 9-fluorenylmethoxycarbonyl (Fmoc) or Tert-butoxycarbonyl (Boc) to prevent unwanted reactions. A coupling agent such as HATU, DIC, or EDC activates the carboxyl group which enables the formation of a peptide bond with the growing chain.
- Deprotection: After coupling, the temporary protecting group is removed, exposing the amino group for the next addition typically done with a mild base like piperidine in dimethylformamide (DMF) with Fmoc or a strong acid such as trifluoroacetic acid (TFA) using Boc.
- Repetition: Steps 2 and 3 are repeated for each amino acid in the desired sequence. This process may require some modifications if certain residues or sequences require specific adjustments to accommodate unique properties.
- Cleavage and Global Deprotection: Once the full sequence is assembled, the peptide is cleaved from the resin, and any sidechain protecting groups are removed simultaneously using strong acids like TFA.
- Purification: Since crude peptides often contain truncated sequences, deletion products, or side products, they require purification through standard methods such as High-Performance Liquid Chromatography (HPLC). Analytical HPLC and mass spectrometry confirm sequence integrity and purity.
Protecting Groups and Chemistry Considerations
Protecting groups are essential in peptide synthesis to prevent undesired reactions. There are different kinds of protecting groups within a peptide synthesis reaction. The amino-protecting groups like Fmoc and Boc protect the N-terminal side of the peptide (1). There are protecting groups for the C-terminal end of the peptide as well, but these are only needed in the liquid phase synthesis. Examples of C-terminal protecting groups include tert-butyl ester and methyl ester. Another group that is commonly added during peptide synthesis is a sidechain protecting group. These prevent unwanted reactions at reactive residues such as serine, threonine, lysine, tyrosine, and cysteine (3). Sidechain protecting groups remain in the reaction through the entire synthesis process and are only removed at the completion of the full chain with a strong acid.
Coupling agents activate carboxyl groups, improving reaction efficiency and minimizing racemization. Common reagents include HATU/HBTU that are efficient for most sequences and compatible with Fmoc chemistry (4). DIC/EDC are often used with additives like Oxyma to reduce side reactions during the synthesis process (4). Optimization of reaction time, solvent, and temperature is crucial for high yields, especially for difficult sequences or those containing bulky or hydrophobic residues.
Challenges in Peptide Synthesis
Sequence Length: Peptides longer than roughly 30–40 amino acids often suffer from incomplete coupling and on-resin aggregation, which reduces yield (1). To overcome this, scientists commonly use segment condensation, pseudoproline dipeptides, or microwave-assisted synthesis to help reactions reach completion.
Hydrophobic and Aggregation-Prone Sequences: Hydrophobic sequences frequently form secondary structures on the resin which can block reagent access and slow down coupling (1). Solvent selection (typically DMF or NMP) along with additives such as chaotropic agents or mild detergents helps maintain solubility and reduce aggregation during assembly.
Sensitive or Reactive Amino Acids: Residues like cysteine, methionine, tryptophan, and histidine are easily oxidized during coupling, cleavage, and storage. For this reason, many protocols handle them under an inert atmosphere such as nitrogen or argon to minimize oxidative side reactions.
Modified Amino Acids: Incorporating phosphorylated and acetylated residues requires specialized protecting groups and optimized coupling conditions. These modifications increase the likelihood of side reactions and often require lower temperatures or longer reaction times to ensure proper incorporation within the synthesis process.
Post-Synthesis Considerations
Purification and Verification: Crude peptides are typically purified by HPLC, using either reverse-phase or ion-exchange columns depending on the peptide’s properties. Mass spectrometry confirms molecular weight, while analytical HPLC evaluates purity. The purity required will be dependent on its downstream use.
Handling: Throughout synthesis, minimizing exposure to moisture and oxygen is crucial, especially for oxidation-prone sequences. Using low-binding plastics and high-quality solvents prevents material loss and contamination, and routinely checking coupling completeness helps avoid errors that can propagate through long sequences.
The Value of Peptide Synthesis
Peptide synthesis is a precise and versatile method for producing custom sequences for research applications. Whether employing solid-phase or liquid-phase methods, the process relies on careful planning, control of chemical reactions, and diligent purification. Challenges can be mitigated through a variety of steps that are needed for each specific synthesis reaction.
Mastering peptide synthesis enables the generation of high-quality peptides tailored to experimental needs. By understanding the elements that provide the optimal results and mitigate risks, laboratories can produce reproducible, biologically active peptides that serve as powerful tools for new innovations and discoveries.
FAQ: Peptide Synthesis
Q: Why is solid-phase peptide synthesis (SPPS) preferred over liquid-phase methods in most research labs?
A: SPPS dramatically simplifies purification because reagents and byproducts can be washed away easily while the peptide remains anchored to the resin. It also allows full automation, faster synthesis, and efficient incorporation of modified or protected amino acids.
Q: How do I know whether a peptide sequence will be difficult to synthesize?
A: Sequences rich in hydrophobic residues, β-branched amino acids (Val, Ile, Thr), or repeated motifs tend to aggregate on the resin and slow down coupling. Long peptides and those containing multiple cysteines or acidic residues also present challenges.
Q: What causes incomplete coupling, and how can it be prevented?
A: Incomplete coupling may result from steric hindrance, aggregation, insufficient activation, or poor swelling of the resin. Increasing coupling time, using more efficient activated esters (HATU), adding pseudoproline dipeptides, or raising reaction temperature can improve yields.
Q: What is racemization, and when does it occur?
A: Racemization is the unintended conversion of L-amino acids to D-forms during activation of the carboxyl group. It occurs most frequently with cysteine, histidine, and serine. Using mild activators like HATU or adding Oxyma helps minimize this issue.
Q: Why do peptides sometimes oxidize during synthesis or cleavage?
A: Amino acids like cysteine, methionine, and tryptophan are sensitive to oxygen and strong acids. Exposure to air or harsh cleavage conditions can generate sulfoxides or disulfide bonds. Many protocols use nitrogen or argon flushing to minimize oxidation.
Q: Why do some peptides fail to dissolve after purification?
A: Poor solubility is often due to high hydrophobic content or strong secondary structure. Adjusting pH, adding mild organic solvents (ACN, DMSO), or using sonication usually resolves solubilization issues.
Q: What is the typical shelf life of a synthetic peptide?
A: Lyophilized peptides stored at −20 °C or below can remain stable for years. Peptides in solution degrade much more rapidly and should only be prepared immediately before use
References
- Bachem. “Solid Phase Peptide Synthesis (SPPS) explained.” June 5, 2023. Available at: https://www.bachem.com/articles/peptides/solid-phase-peptide-synthesis-explained/
- Neuland Labs. “Liquid Phase Peptide Synthesis: Guide to Methods, Use Cases, & Strategic Advantages.” July 30, 2025. Available at: https://www.neulandlabs.com/en/insights/stories/liquid-phase-peptide-synthesis
- Biosynth. Protecting Groups in Peptide Synthesis. Available at: Here
- Thermo Fisher Scientific. “Peptide Design: Principles & Methods.” Protein Biology Resource Library. Available at: https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/peptide-synthesis.html



